Omega-3
So, what’s the difference between pills? (Part 2.)
The Innovator Pathway FDA-approved innovative prescription products – These are unique new drugs (chemicals or biologicals) that have undergone years of investigation and scrutiny by the FDA, and scientific and medical communities. Sadly, the rules for marketing approval that apply to these new chemical or biological agents aren’t concrete and vary from product to product…
Read MoreSo, what’s the difference between pills? (Part 1.)
What Government Regulations Control Drugs, Supplements, and Medical Foods in the United States As you walk through pharmacies, the number of items that look like medicine is daunting. There are literally hundreds of tablets, capsules, liquids, topicals, and injectable products available to the public, but few people understand how these products are controlled for safety…
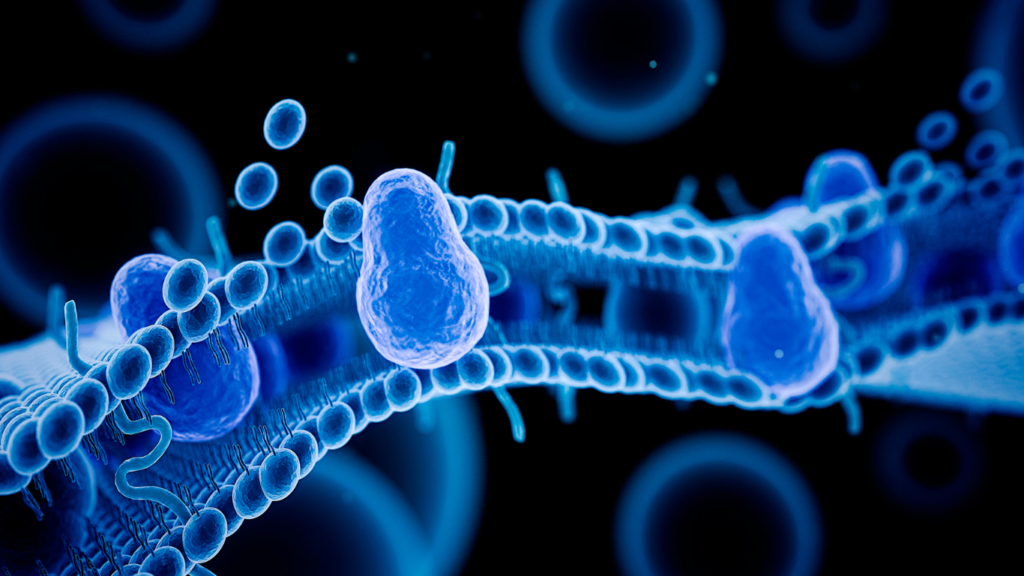
Read MoreThe Role of Cell Membranes in Health: Unveiling the Hidden Secrets
Introduction: Every living organism, whether it’s a human being or a plant, is composed of fundamental building blocks known as cells. These microscopic entities share standard components, with one crucial element being the cell membrane. The cell membrane serves as a guardian, keeping the inner contents of cells safe while preventing unwanted materials from entering.…
Read More